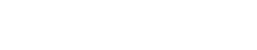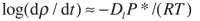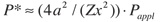Part 1 of 3: Development and application of polycrystalline diamond compact bits have overcome complex challenges from the difficulty of reliably mounting PDC cutters in bit bodies to accelerated thermal wear.
Federico Bellin, Alfazazi Dourfaye, William King and Mike Thigpen, Varel International
Polycrystalline diamond compact (PDC) cutters and bits have been a significant contributor to the greatly improved efficiencies and economics of oil and gas drilling over the last 30 years. This article—the first of a series of three on the evolution, current state of the art and long-term outlook for this technology that has revolutionized the drilling industry—will cover the technology’s early commercial history and technical challenges, and will address the major issues encountered in PDC cutter manufacturing.
ADVENT OF PDC TECHNOLOGY
Polycrystalline diamond compact cutters, referred to at the time by the trade name Stratapax, were first developed by General Electric in 1973. They utilized GE’s earlier invention, monocrystalline manmade diamond, which was reloaded into a pressure cell with a tungsten carbide substrate and re-pressed to produce a compact of 13-mm diameter and 3.3-mm length that incorporated a 0.5-mm-thick diamond table. In the press cycle, cobalt in the substrate would sweep into the diamond crystals and act as a catalyst to produce diamond-to-diamond bonds resulting in the creation of a polycrystalline diamond table bonded to the tungsten carbide substrate. GE mounted the new cutting elements onto longer tungsten carbide cylinders, or alternatively onto tungsten carbide posts, and set about marketing the capabilities of PDC through the commissioning of test bit production for lab and field testing.
PDC drill bits first found very limited commercial applications in oil and gas drilling in the late 1970s. Early areas of success included South Texas and the North Sea. Dr. William C. Maurer’s 1980 text Advanced Drilling Techniques included an entire chapter titled “Stratapax bits” on PDC cutters and bits that summarized the state of the art for this new cutting element and its potential for drilling. The concluding paragraph of the chapter reads as follows: “Tests to date indicate that STRATAPAX bits have potential for significantly reducing drilling costs in the mining, geothermal, and petroleum industries. Improved STRATAPAX bits would allow increased use of high speed drilling motors which would further increase drilling rate and reduce drilling costs. Because of this high potential payout, R&D on STRATAPAX bits should be significantly increased.”
Research and development expenditures indeed followed, including further efforts by GE. In addition, the traditional natural diamond and roller-cone drill bit companies increasingly devoted efforts to the commercial application of PDC in drilling. They were joined by startup PDC bit companies including Davis & Hicks and Stratabit. Even with all the early focus given to PDC bits, by 1982 they still were responsible for less than 2% of all footage drilled.
EARLY TECHNICAL CHALLENGES
In the three decades since 1980, the development and application of PDC bits has moved forward in steps that sequentially overcame the most immediate challenges of the time. The first difficulty was reliably mounting PDC cutters in bit bodies. Brazing techniques and practice of the day frequently led to debilitating cutter loss and failed runs. Post-mount press-fit cutters that were deployed in steel-body bits were prone to fracture breakage of the post at the mounting point and to loss through erosion of the steel bit body. Improved brazing and mounting pockets provided solutions.
Early PDC application guidelines required oil-based drilling fluids, especially in shales, limiting the products’ market potential. Bladed bit designs with deeper junk slots and greater open face volume combined with improved jet nozzle hydraulics experience opened up economic applications in water-based fluids as well.
Mitigation of bit whirl. The next major hurdle was overcoming the effects of impact damage to the PDC cutter. This type of damage was typically manifested by diamond table delamination from the cutter substrate, escalating to cracking or breakage of cutter substrates, and frequently culminating in gross breakage of cutter pockets and bit blades.
The initial solution to this problem was the development of non-planar diamond-to-substrate interfaces. These consisted of grooves and other patterns on the face of the tungsten carbide substrate that allowed for a transition zone between the extremely stiff diamond table and the somewhat more flexible substrate. These interfaces resulted in reduced diamond table delamination.
At this stage of development, PDC bits had increased their market presence to about 15% of all footage drilled and were considered by many to have neared the peak of their potential development. A major advancement, however, was made by Amoco’s recognition of the phenomenon of “bit whirl” in the late 1980s. This self-regenerating off-center rotation condition caused PDC drill bits to experience high lateral forces that were responsible for much of the run-ending impact damage seen on many dull bits, especially where harder transitional layers were encountered in otherwise PDC-drillable rock columns. Over the next several years, the PDC bit design community developed technologies and methods to mitigate bit whirl, including force balancing, blade asymmetry, blade and gage spiraling, cutter tracking, smooth gage configurations and penetration limiters.
These developments increased the potential for the economic application of PDC bits and drove the next development: improved rock analysis tools to better program and apply PDC bits. Predictive lithology analysis coupled with prescriptive operating parameter plans increased the efficiency and economic success rate of PDC bits. By the late 1990s, PDC bits accounted for about 45% of all footage drilled in the oil field.
Resistance to abrasive wear. With earlier challenges including bit balling, cutter loss, impact damage and improper programing all mitigated, the next challenge was to improve the attribute most sought after in diamond, the resistance to abrasive wear. PDC cutters were subject to thermal damage and accelerated wear at the cutting tip, due in large part to the residual cobalt catalyst remaining in the interstitial matrix of the cutters’ polycrystalline diamond face. By reducing the cobalt content in the outermost layer of the diamond table, the cutters’ abrasion resistance and thermal stability were significantly improved, allowing PDC bits to compete economically with roller-cone bits in even more applications.
In 2010, PDC bits account for an astounding 65% of footage drilled in oil and gas applications and still do not appear to have peaked in their development. More research than ever is going into PDC bits, and especially into PDC cutters.
PDC MATERIALS AND PROCESSING
Polycrystalline diamond compacts are produced by sintering diamond grit with a catalyst in an ultra-high-pressure and high-temperature process. PDCs are among the most rigid of all diamond tool materials. They exist in various structures and shapes, but generally for oilfield drill bits, a layer of polycrystalline diamond is atop a cylindrical tungsten carbide substrate.
Making the diamond. Since GE manufactured the first manmade diamond in 1954, the technology has spread around the world. Increasingly large high-pressure/high-temperature (HPHT) tools have been developed. Even so, the concepts and even the materials used to make the HPHT cells are basically the same. The two main press technologies currently used to produce virtually all synthetic diamond powder and sintered polycrystalline diamond (PCD) are the belt press and the cubic press. Other technologies exist, but their use is limited to research and development, where very high pressures and temperatures are achieved on tiny samples mainly for geology studies.
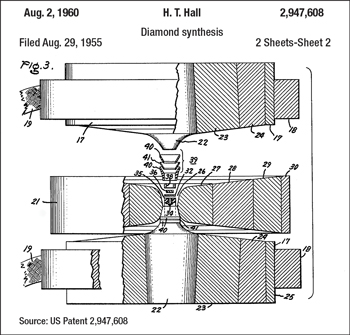
The belt press concept was the first, developed in the 1950s when GE managed to grow diamond crystals for the first time in history, Fig. 1. Modern systems are not too different from the initial 1954 design; the main improvements consist of changes in the cell size increment and the development of HPHT cell materials with better properties and consistency.
 |
|
Fig. 1. The cutaway diagram of the original GE belt press.
|
|
Usually, the two conical anvils and the die are made of hard metal such as tungsten carbide-cobalt (WC-Co), while the binding rings, which are mounted with increasing mechanical interference, are made of high-strength steel.
A load is applied axially to the top and bottom anvils, which are pushed inward against the high-pressure cell placed inside the die. The cell’s ceramic material is squeezed out into the gap between the die and the anvil flank surfaces, providing a seal against the increased pressure within the cell. An electric current is then flowed through a graphite heater to raise the temperature of the inner portion of the cell to begin the sintering process.
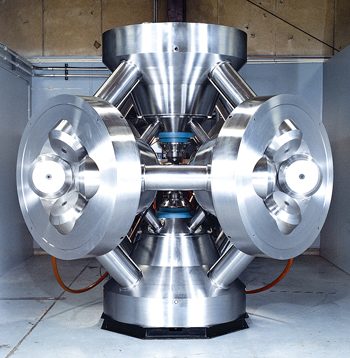
Cubic press technology was originally developed as an alternative method of generating the diamond synthesis conditions. The cell has a cubic shape, and six hard metal anvils are pushed against the six cube faces, Fig. 2. In this type of press, also, the edges of the ceramic material are squeezed out into the gaps between the anvil faces, providing the sealing effect in the same way the belt press does. Again, force is applied and the temperature altered to begin diamond sintering.
 |
|
Fig. 2. A modern cubic press. Courtesy of US Synthetic.
|
|
When designing and operating an HPHT system, the challenge is to reach the 800,000 psi and 2,600°F required for sintering while maximizing the life expectancy of the expensive hard metal tools used as anvils and dies. PCD and synthetic diamond manufacturers are constantly striving to improve the performance and the cost-effectiveness of their HPHT systems, so more extreme sintering conditions can deliver the next generation of high-performance drilling products.
Pressure. In traditional PCD manufacturing, pressure and temperature are two critical variables due to their importance in determining the final properties of the sintered diamond product and also for the technical challenges involved in designing the HPHT apparatus.
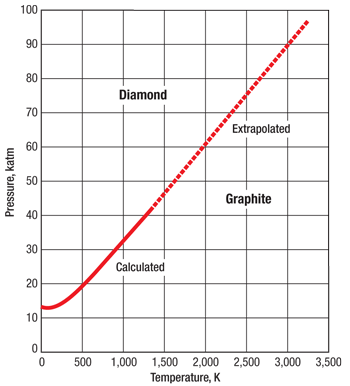
At atmospheric pressure, diamond is not in a thermodynamically stable phase, but graphite is. The fact that diamonds are not spontaneously converting to black carbon at room pressure and temperature is due to the fact that the speed of the reaction is virtually zero. If the temperature surpasses 2,200°F, however, the reaction pace will increase, leading to the spontaneous conversion of diamond to graphite. To make diamond, or to sinter it, it is necessary for the conditions to support the thermodynamically stable phase of diamond rather than graphite. In other words, we need to increase pressure together with temperature to speed up the synthesis, or bonding, process. Figure 3 shows the graphite-diamond equilibrium curve, according to which every 100°F temperature increase requires a pressure increase of 14,500 psi to remain in the diamond stable range. It is also clear that the need for a fast reaction (and, thus, high temperature) is in conflict with the manufacturing necessity of working at lower pressures to extend the life of the high-pressure tools.
 |
|
Fig. 3. The graphite-diamond equilibrium curve.
|
|
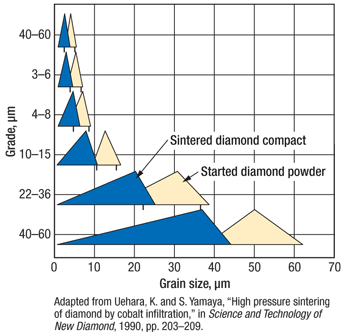
Usually the diamond powder sintering process consists of two steps. First, pressure is raised to its nominal level with little or no heating. During this stage, all the crystals are being pushed against each other with increasing force. Many diamond particles are sliding relative to each other and many are cracking into two or more fragments with the overall effect of increasing the powder apparent density. The powder crushing caused by the pressure increase can be easily quantified by measuring the particle size distribution before and after a full-pressure cold run, Fig. 4. Interestingly, a coarser powder presents a higher degree of crushing than a finer one. This fact can be easily explained in terms of average number of contact points per unit volume (much higher for fine powders), thus lower contact stress and lower probability for a small particle to fail.
 |
|
Fig. 4. Grade-to-grain size chart.
|
|
Second, when the crushed and compacted powder is under full pressure, the temperature is raised to its nominal value. The diamond powder is usually packed against a cobalt (WC-Co) substrate, which is the source of the catalyst metal that promotes the sintering process. When the cobalt reaches its melting temperature of 2,615°F at 841,200 psi, it’s instantaneously squeezed into the open porosity left in the layer of compacted diamond powder. At this point, the sintering process takes place through a mechanism of carbon dissolution and precipitation—the driving force being the reduction of the overall internal energy. Technically, this process is defined as a pressure-assisted liquid-phase sintering. The driving force for the densification under an external pressure is determined by the pressure itself and also by the contact area relative to the cross-sectional area of the particles. The reaction speed is proportional to the temperature and to the average effective pressure P*, which is the actual contact pressure between particles, as expressed in the equation:
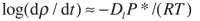
where r is the powder apparent density, Dl is the carbon diffusivity in the molten metal catalyst, R is the ideal gas constant and T is the temperature. As the equation shows, the sintering process is faster if we increase both the contact pressure and the temperature. The contact pressure P* can be expressed as:
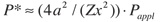
where a is the average particle size, x is the radius of the contact area between two spherical particles, Z is the number of surrounding particles and Pappl is the external pressure applied to the system. Clearly, smaller grain size and better packing result in lower contact pressure; thus, when sintering PCD grades with small average grain sizes, higher pressures and temperatures are usually required.
Grain size. A single diamond crystal is extremely hard and has a very high wear abrasion resistance and thermal stability, but it is a highly anisotropic material: Its properties are different depending on the plane in which they are measured. This allows the natural gemstones to be cut along specific “cleavage” planes where the energy required to split the crystal is at its minimum. In a diamond sintered compound, all the weak planes are randomly oriented so, at a macroscopic scale, the material will behave isotropically with improved impact strength.
When designing a novel PCD grade for drilling applications, it is possible to mix together diamond powders with different average particle sizes and dimensional statistical distributions. As a rule of thumb, it can be assumed that the smaller the size of the crystals sintered together, the higher the wear abrasion resistance—at the expense of lower impact strength. The opposite is true when the diamond powder recipe includes coarser starting powders even if, according to some studies, increasing the average grain size above 50 µm doesn’t significantly improve PCD toughness. The lower limit in terms of powder size is set by manufacturing issues that can be summarized as a) increasingly high pressures required to consistently sinter the tiny diamond crystals and b) difficulties in controlling the grain growth during the process.
It is worth mentioning the importance of the particle size distribution besides the crystals’ average grain size. Mixing particles with a wide range of dimensions is important to achieve a good degree of powder packing: Minimizing the empty spaces between crystals favors a good sintering process during the HPHT cycle, delivering a PCD with superior toughness and wear abrasion resistance. A lot of research and development resources are dedicated to the development of new diamond powder recipes with the objective of maximizing both wear abrasion and impact strength.
Carbide interface. The interface between the sintered diamond table (PCD) and the WC-Co substrate is a critical feature of a shear cutter: Not only does it have to provide the necessary strength so the insert can manage the static and dynamic shear loads that otherwise would cause the diamond table to delaminate, but it also has to handle the residual stresses that arise within both the substrate and the PCD as a consequence of the HPHT sintering process. To accomplish this objective, it is a common practice to design special non-planar interfaces (NPIs) that increase the amount of available carbide surface to which the diamond table can attach. Residual stresses are generated during the sintering process—more specifically, during the cooling stage when the PCD is already fully sintered. The thermal expansion coefficient mismatch between the PCD layer and the substrate causes the carbide to shrink more than the top diamond table, forcing it to bend outward.
The tensile state of stress within the diamond becomes worse as its thickness increases, leading in some instances to a spontaneous delamination of the top layer. Extensive use of finite-element analysis tools is required to simulate the residual stresses’ field distribution for different combinations of diamond layer thicknesses and different interface geometries. It is a good practice to avoid the repetition of regular geometric patterns, and instead to resort to a random distribution of geometric features with varying shapes and dimensions. Many NPI designs have been tested over the years, and many variations are available.
Grit quality. Commercially available PCD material composition is up to 98% diamond in volume, the balance being the catalyst metal that has infiltrated from the substrate. For this reason, the quality of the feedstock diamond powder quality is of utmost importance in determining the final properties and consistency of the sintered product. Usually, the micronized diamond powder used in PCD manufacturing is a byproduct of the industrial diamond powder synthesis process, where crystals of at least 100-µm size are produced mainly for the stone cutting market (e.g., diamond saw blades, diamond wheels, loose diamond powder). The two main catalytic systems used for the diamond synthesis are based on cobalt and on an iron-nickel alloy.
The industrial diamond powder available on the market has an extremely wide quality range. The micronized powders used in the sintering process are usually made by crushing coarser ones, so the quality of the initial industrial powder affects that of the shear cutter feedstock crystals. Depending on the press cycle parameters (mainly pressure and temperature), crystals can be grown with different shapes (e.g., cubic rather than octahedral). Furthermore, if the crystals are growing too fast within the molten catalyst bath, it is possible to find metal inclusions buried deep inside the crystal at the end of the cycle.
Other quality issues concern the diamond powder extraction and cleaning process from the solidified alloy catalyst in which synthesis took place. Usually this is done by dissolving the metal in a hot bath of hydrochloric and nitric acid, then rinsing and cleaning to remove any residue left by the previous acid dissolution step. If this process is not performed thoroughly, the diamond powder surface will be contaminated and the sintering process will not be as effective, leading to PCD performance and quality issues.
NEXT INSTALLMENT
Part 2 of this series will present an extensive discussion of PDC cutter leaching, including the advantages of leaching and how the effects of a leached diamond layer can be modeled. Also, the testing and qualification processes for PDC cutters will be reviewed in depth, including both destructive and non-destructive testing methods. 
ACKNOWLEDGMENTS
The authors would like to thank Varel International for permission to publish this article series. Thanks also to Diamond Innovations, US Synthetic, Dennis Tool, the Paris School of Mines, the University of Trento, Mike Reese, Crystal Montanez and Brandi Williamson for their efforts in developing the series.
|
THE AUTHORS
|
| |
Federico Bellin is a Senior Technology Engineer for Varel International. He has 12 years’ experience in PDC cutter and insert design and is highly experienced in HPHT technology. Mr. Bellin has worked both in Europe and the US cooperating with R&D institutions worldwide to develop new materials and new designs for ultra-HPHT systems. He earned a master’s degree in materials science in 1997 from the University of Trento, Italy.
|
|
|
| |
Alfazazi Dourfaye is the Manager of Technology Development for Varel International. He has 20 years of experience in PDC cutter and bit technology including testing development, bit applications and engineering, and software development. Dr. Dourfaye has developed a thermal mechanical model of PDC cutter wear and a simulator of PDC bit performance monitoring with cutter wear. He graduated from the Alès School of Mines in 1990 and earned his PhD at the Paris School of Mines in 1995. |
|
|
| |
William King is the Director of Marketing and Intellectual Property for Varel International. He has 29 years of experience in the drill bit industry, including roles in design, product development, bit applications, international sales, software development, marketing and intellectual property management. Mr. King is the inventor or co-inventor of 15 issued and five pending US patents. He attended the University of Illinois and the University of Utah. |
|
|
| |
Mike Thigpen is a Senior Technology Engineer for Varel International. He has 26 years of experience in the oilfield service industry including roles in design, product development, bit applications and engineering management. Mr. Thigpen is the inventor or co-inventor of nine issued and two pending US patents. He earned a BS degree in mechanical engineering from the University of Houston in 1983. |
|