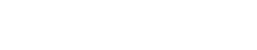VOC/HAP emission limits in the Rockies: Challenges and controls
Separation technologies are unable to remove dissolved hydrocarbon compounds that may represent a large portion of volatile organics.
Separation technologies are unable to remove dissolved hydrocarbon compounds that may represent a large portion of volatile organics.Robert Schlemmer, Scomi Oiltools, Kuala Lumpur, Malaysia; Volatile organic compound (VOC) and hazardous air pollutant (HAP) emissions from produced water surface disposal facilities are of regulatory and public concern in the Western US. More stringent air emission regulations present challenges to owners and operators of surface disposal facilities, including decreased profitability, especially in the more intensely populated areas. Currently available and emerging engineering controls may reduce or destroy VOCs and HAPs before their release into the atmosphere. Adoption of these control technologies could increase produced water disposal facility profitability and potentially generate enough revenue to cover or partially defray their capital cost. INTRODUCTION Produced water is generally defined as water extracted from the earth’s subsurface during production of oil and gas. This water may include geologic formation water, injection water and chemicals added to the formation to stimulate production, e.g., frac water, or water and/or chemicals added to improve oil-water separation,1 Fig. 1.
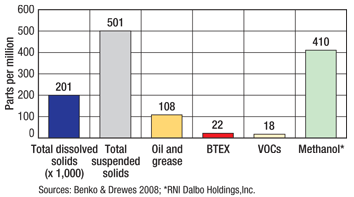
Produced water represents the largest waste stream volume generated by the oil and gas industry during E&P. Produced water volumes are generally three or more times the volume of crude oil extracted from a given well.2 Each year, the US generates about 21 billion bbl of produced water. The Western US produced 3.1 billion bbl in 2007.3 Current practices of produced water disposal in the US include reinjection into the subsurface via injection wells and surface discharge (such as evaporation ponds, reuse and land application).4,5 The five Rocky Mountain states (Colorado, Utah, Wyoming, New Mexico and Montana) have seen a rapid increase in the number of disposal facilities using evaporation ponds. This observed increase presents environmental compliance challenges to both regulators and facility owners. The growth in private and commercial produced water disposal facilities in the Rockies has been fostered by increased oil and gas development, as well as the relatively dry climate, availability of low-cost land, and historically low costs to operate and maintain evaporation ponds. Operating costs range from $0.01 to $5.50 per bbl of produced water, including the cost for water hauling.2 A typical private or commercial produced water disposal facility using evaporation ponds in the Rocky Mountain region includes: At the facility, oil-water separators employ gravity methods to separate the immiscible oil and solids. Recoverable hydrocarbons typically range from 1% to 2% by volume of the bulk produced water. Vacuum trucks, skimmers and overflow weirs are employed to collect oil and condensate from gravity separation vessels for temporary storage in tanks before shipping to market or further processing. Primary and secondary separator units and evaporation basins cause VOCs and/or HAPs present in the produced water to escape into the atmosphere. VOC/HAP EMISSION RULES IN THE ROCKIES Colorado, Utah, Wyoming, New Mexico and Montana regulate VOCs and HAPs as outlined in Table 1. Native American tribal lands in these states are regulated directly by the Environmental Protection Agency (EPA) and are outside the scope of this article. Most states have limits on the amount of VOCs and/or HAPs that can be discharged into the atmosphere, require specific water sampling of certain hydrocarbons and additives (e.g., methanol), and impose fees to emit and fines for violators. In addition, some counties in Utah and Colorado (e.g., Grand County, Utah, and Mesa County, Colo.) have adopted county regulations for further limiting VOC/HAP emissions.
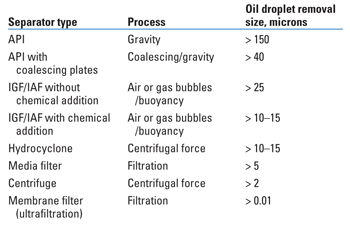
VOC AND HAP IN THE ENVIRONMENT The fate and transport of total petroleum hydrocarbons (TPH), including benzene, toluene, ethelbenzene and xylene (BTEX) and n-hexane, and methanol in produced water affects VOC and HAP emissions in the atmosphere as well as the mechanisms that decrease TPH and methanol volatilization. In general, TPH in produced water may exist as: A portion of TPH floating on the water surface or dispersed in the bulk water can be readily separated from the bulk water using specific gravity differences or other physical processes. The volatile portions of dissolved TPH may escape into the atmosphere through volatilization. Sorbed TPH may form particle coatings and may settle out with sediments.6 Other mechanisms (based on the chemistry of the bulk water) may reduce the amount of TPH and, in turn, VOCs and HAPs in produced water. These may include:6,7 These mechanisms, in concert with temperature changes, oil film thickness, water retention time, diffusion and convection mechanisms, evaporation basin depth, and differential water solubility of hydrocarbons, may reduce formation and mass transfer of VOCs and HAPs to the atmosphere.8 Methanol has been included in the volatile VOC compounds list and is classified as an HAP; however, methanol is very soluble in water (infinite miscibility) and tends to remain dissolved, as shown by its low Henry’s constant (i.e., 0.0027 atm L/mol).7 VOC/HAP ESTIMATION METHODS Many states in the Rockies use a VOC/HAP emission estimation method by which TPH, BTEX, methanol and other chemical concentrations in water are assumed to volatilize completely. Several alternative approaches for estimating VOC emissions are approved or are being evaluated by the five Rocky Mountain states and the US Environmental Protection Agency (Region 8). The Colorado Department of Public Health and Environment (CDPHE) has accepted the EPA AP-42 (Chapter 4.3) “waste water” method.8,9 Also, both the Utah and Wyoming Departments of Environmental Quality approve air canisters as VOC and HAP emission sampling methods. CDPHE does not currently approve air canisters as an alternative VOC estimation method; however, it allows flux chambers for estimating fugitive VOC emissions from open water bodies, given prior approval of methods and procedures. Remote sensing is an emerging technology that oil and gas refineries have widely used for detecting fugitive emissions.10 This technique is currently being evaluated by EPA (Region 8). ENGINEERING CONTROLS Some facilities have successfully employed many commercially available engineering controls for recovering and reducing VOCs and HAPs. Generally, engineering controls include a single treatment control or a combination of controls that fall into one or more of four categories based on the process used:11 physical, chemical, biological and thermal. Physical processes rely on physical means such as gravity (driven by density differences, oil droplet size, etc.) and filtration to separate oil and water. The physical separation technologies (conventional gravity separators and enhanced gravity separators) can only recover free-floating and dispersed oil from the produced water. Conventional oil-water separators may include tank-type separators (e.g., gun barrel) and API-type separators (rectangular, square or circular in shape) with or without design modifications such as coalescing plates. Design modification in an API separator can increase separation efficiency by increasing coalescence rates of oil droplets, which can enhance the rise rate of oil droplets and require a shorter residence time, and, in turn, a smaller system footprint.1 Table 2 lists the oil-removal capabilities of different types of oil-water separators by droplet size.12
Conventional API separators are more effective at separating oil droplets larger than 150 µm, whereas more energy-intensive processes, such as hydrocyclones, can remove oil droplets greater than 10–15 µm in diameter. Enhanced gravity separators include: Chemical separation technologies use chemical sorption processes or air stripping techniques to transfer oil or VOCs from one phase to another—for example, liquid to solid or gas to liquid. Biological processes (i.e., bioreactors) using microbes or bacteria are also employed to transform or biodegrade toxic organic compounds to less- or non-toxic organics. Abatement technologies may employ thermal-oxidizers, flares, chemical agents or heater treaters to destroy VOCs and HAPs. These methods, although effective in reducing VOCs, may destroy or otherwise reduce the volume of valuable commodities that could be recovered and sold for profit. DISSOLVED OIL REMOVAL TECHNOLOGIES The separation technologies previously described can effectively separate dispersed and free-floating oils. They are, however, unable to remove dissolved hydrocarbon compounds that may represent a large portion of VOCs. One technology to accomplish this uses chemical coagulation of dissolved hydrocarbon molecules by binding them to like molecules embedded in the media filter. The chemical affinity between the binding molecules and hydrocarbons (including semi-volatile organics) produces a water-repellant mass that is subsequently removed from the filter. This technology has been used with varying degrees of success in Utah by Anadarko to reduce dissolved hydrocarbon concentrations to 10 ppm before disposal in evaporation ponds.13 Another separation technology uses conventional oil-water separation in combination with a vapor compression, turbulent flow and flash evaporation treatment system. The system removes and recovers marketable hydrocarbons and alcohols. It produces clean, distilled water for use at drill sites for completion fluid makeup, surface drilling or discharge directly into surface water. Operators in the Pinedale anticline of Wyoming have used this technology to meet or exceed established surface discharge water-quality standards.14 In conclusion, there are a number of proprietary enhanced separation methods currently on the market. Some of these are effective for specific applications, but no individual technology provides a universally applicable method for oil recovery and/or VOC reduction. Engineering controls should be implemented on a case-by-case basis. ACKNOWLEDGMENT The authors thank the Wyoming Department of Environmental Quality, the Utah Department of Environmental Quality, the New Mexico Department of Environmental Quality, the Montana Department of Environmental Quality, the Colorado Department of Public Health and Environment and Grand County, Utah, for providing useful regulatory information. LITERATURE CITED 1 Dicataldo, G., Richins, G. H., Spiroff, K. J., Nelson, M. B., “Produced-water VOC, HAP emissions concern Rocky Mountain regulators,” Oil & Gas Journal, 107, 2009 (25), pp. 41-50.
|
||||||||||||||||||||||||||||||||||||||||||
- Shale technology: Bayesian variable pressure decline-curve analysis for shale gas wells (March 2024)
- Prices and governmental policies combine to stymie Canadian upstream growth (February 2024)
- U.S. producing gas wells increase despite low prices (February 2024)
- U.S. drilling: More of the same expected (February 2024)
- U.S. oil and natural gas production hits record highs (February 2024)
- U.S. upstream muddles along, with an eye toward 2024 (September 2023)
- Applying ultra-deep LWD resistivity technology successfully in a SAGD operation (May 2019)
- Adoption of wireless intelligent completions advances (May 2019)
- Majors double down as takeaway crunch eases (April 2019)
- What’s new in well logging and formation evaluation (April 2019)
- Qualification of a 20,000-psi subsea BOP: A collaborative approach (February 2019)
- ConocoPhillips’ Greg Leveille sees rapid trajectory of technical advancement continuing (February 2019)