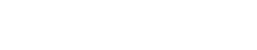PRODUCED WATER REPORT
Troubleshooting produced water-chemical testing and recommendations
Part 2 of 2: A task group uses its experience on an FPSO to form a general produced water troubleshooting methodology.
John Walsh, John Fanta, William Bryson and Celso Toschi, Shell; Joseph Lee, Natco Group Inc.; Ted Frankiewicz, SPEC Services Inc.; John Petty, Modec International, LLC; and Melvin Stacy, CCR, LLC
This is the second and final part of a report on the troubleshooting efforts of a Produced Water Task Group that was assembled to address problems on an FPSO. While water production at the FPSO was still only one quarter of the nameplate capacity, a significant fraction of the treated produced water did not meet discharge quality requirements and had to be diverted to cargo tanks for later processing. In the cargo tanks, the oily water remained in an emulsified state. After several months of problems, the cargo tanks were beginning to fill, leaving no way to continue production at existing rates. The task group was formed to develop facilities, equipment and operations-based solutions.
Part 1, in March, gave a summary of the system performance and an overview of the problems that were encountered in the field. The current article details the methods used to troubleshoot the problems, the short-term and long-term recommendations and the initial results of the changes that were made.
BACKGROUND AND REVIEW
In 2003, Shell assumed ownership of an FPSO as part of a resource acquisition. Shell had no input regarding the design of the facility. In late 2004 and early 2005, water production began to increase, in part because of early seawater breakthrough from the waterflood. The rate of water production (about 12,000 bwpd) was far below the FPSO"s 50,000-bwpd design rate. Nevertheless, water- and oil-treating problems were consuming significant operator effort. Increasingly, the water-treating system was unable to treat the produced water to acceptable levels of oil and grease. When the oil and grease of the produced water was higher than acceptable, the produced water would be diverted from the overboard discharge line to one of various cargo tanks for later processing. Water was being diverted inboard with increasing frequency, and the cargo tanks were filling up. In addition, pads were forming in the separators, which had to be cleaned out with increasing frequency.
Part 1 presented a process description and the initial conclusions from field studies. To summarize these results, the following problems were identified:
- The facility is not designed to handle produced fluids that contain high loads of fine solids
- Problematic recycle streams exacerbate existing emulsion problems
- There is no wet-oil tank or slops-treating system to treat the solids-laden oily water.
Process Routing. A particular focus of Part 1 was the process-routing problems encountered on the FPSO. In brief, these were:
- The upstream separator design results in the periodic discharge, probably due to wave motion, of significant volumes of oily emulsion and free oil directly into the water-treating system
- The water-treating system is not designed to handle these discharges
- Internal recycle streams, including treating chemicals and oily solids, are routed through pumps and control valves and fed into the oil train, where they contribute to pad formation in the vessels and further decrease separation efficiency
- There is no slops or oily-water-treating system and no route for the treatment of solids-stabilized emulsions or for the discharge of solids; thus the solids become trapped in the system in a closed recycle loop
- The reject streams, when operated at proper design reject ratios, are much greater than design assumptions
- The IP separator and the BOTs lack interface bleed systems; pads build up frequently, and the only means to dump them is through a route that contaminates a large volume of produced water, diverting it inboard to the cargo tanks.
Recommendations for ameliorating or solving some of these problems were discussed in Part 1. Other short- and long-term solutions are discussed in this article.
METHODOLOGY
The E&P industry is seeing an increase in produced-water-treating problems, and guidance is needed in the literature on how to troubleshoot such problems. While some case studies exist, the complexity of produced-water treating demands more research.1 Water treating is a complex subject as many process variables can affect water quality. Which of these variables has the most effect, or which could be modified with the greatest benefit-cost ratio, is usually not immediately apparent. This is where experience and case studies can help.
Another complicating factor is that the underlying science of water treating involves colloidal chemistry in general, and emulsion science in particular. These are specialized areas, and many, if not most, of the staff involved in the E&P industry are not exposed to these subjects through their educational background or workplace training. Without such knowledge, it is difficult to recognize quickly the cause of water-treating problems. On the other hand, field experience can greatly supplement a lack of fundamental knowledge. Where direct field experience is lacking, a good rapport with the operators is beneficial. All things considered, there are many ways to approach a water-treating problem.
One of the major challenges in troubleshooting produced-water problems is to differentiate between chemistry-related problems (e.g., fluid compatibilities, formation solids, precipitated solids, water soluble organics, natural and synthetic surfactants) and mechanical problems (e.g., sources of shear, process bottlenecks, poorly tuned process parameters, vessel internals, plugging/fouling of equipment, process recycle streams and system dynamics). In many instances, the problem areas overlap. Furthermore, a mechanical problem may be partially ameliorated by optimizing the chemical program. As a first step, though, there is benefit in understanding the underlying cause of the problem.
It must be pointed out that there is no “correct” topsides design that will provide good water quality for all fluids. The only practical way to design and build a topsides facility is to understand the fluid properties and the available design options, and to make appropriate compromises.
Topsides problems are most often due to a mismatch among the fluid properties, the chemical treating system employed and the compromises that have been made in vessel and system design.
As in any troubleshooting methodology, it is important to develop a well-defined problem statement. The problem statement must be factual and accurate. If it is based on false assumptions, the troubleshooting project team will start off in the wrong direction and waste valuable time. If the problem statement is based on observations or data, these must be verified initially.
Once a problem statement is developed, the issues typically investigated fall into four broad and overlapping areas:
- Produced fluid properties
- Chemical treating program
- Process routing, vessel capacities and internals
- Operating practices.
Table 1 lists specific items that should be considered and questions that should be answered in each of these four areas to determine the sources of problems. This methodology is not exhaustive, but it has been demonstrated to be helpful. We do not offer “correct” answers to these questions since the correct answer to one question will often depend on the answer to another question. Also, a treating system that works well for one fluid may not work well for another. An acceptable operating practice for one platform, with its particular fluids and equipment, may not be acceptable for another.
| TABLE 1. Areas to check when troubleshooting produced water problems |
 |
|
Another very effective tool in troubleshooting water-treating problems is to determine the technical limit of the equipment and to compare the technical limit with operating performance. This can help determine if vessel internals are poorly designed, fouled, broken or otherwise not performing properly. Also, it can help identify poor operating practices. Without going into details, there are two steps in determining the technical limit, which can be taken in parallel. One step is to work with equipment designers to determine the theoretical expected efficiency. The designers have design and performance curves that can be used to determine the theoretical peak performance of the equipment. The more sophisticated vendors will also have the capability to perform computational fluid dynamics, which not only will help establish the theoretical peak performance but will also give a measure of the expected benefit of installing new vessel internals.
The second step is to carry out field measurements of efficiency (e.g., from BS&W and total oil and grease measurements) and to determine the reject ratio (from inlet, product outlet and reject outlet) of particular process operations. If flowmeters are not available to determine all the necessary flows, they can be estimated by a mass and material balance calculation. Conducting tracer studies can also help to determine if streamlining, short-circuiting or skateboarding is occurring in the vessel. In addition, if there are any parallel units in the facility, a simple and effective means of finding problems in one vessel is to compare the operation of all units in a parallel train.
Applying the technical-limit-related troubleshooting steps to the FPSO of interest revealed that one of the primary inlet separators was operating at significantly less than peak performance. Upon a subsequent FPSO shutdown, it was discovered that one of the baffle plates in that vessel was damaged. Upon repair of that plate, and subsequent startup, vessel performance improved.
The material below discusses some of the prominent findings that emerged as we followed this troubleshooting methodology for the FPSO of interest. Process routing having been discussed in Part 1, the material focuses on the other three areas of the troubleshooting methodology.
PRODUCED FLUID PROPERTIES
The oil properties did not appear to be an exceptional challenge. There were two primary reservoirs with similar fluid properties. The oil density was 27° API. The asphaltene content was roughly 5% and 7% in the two reservoirs, with 12% and 14% resins, respectively. In addition, the aromatic content of the oil was roughly 40%, making the overall character of the oil relatively good as an asphaltene solvent. Thus, from the standpoint of the oil properties, asphaltene stability was not a problem. This was verified in the field. The operators indicated that asphaltene precipitation had not been observed in the vessels upon vessel entry during a recent shutdown. On the other hand, given the relatively high asphaltene content, some asphaltene precipitation—if not deposition—was still likely and could contribute to emulsion stability.
The produced fluid (oil and water) was sampled at the primary separators and tested onshore for solids content. The intent was to quantify the small particles that would contribute to emulsion stability. Testing was carried out according to ASTM 4807-D, using a 0.45-micron filter, and the results are reported in lb/1,000 bbl oil or ppt, Fig. 1. The fields of interest (designated "B" and "S") were compared with data from other locations worldwide, including some fluids with high solids contents that are known for being challenging to demulsify. While the B and S fields are ranked sixth and seventh, they have only slightly lower solids content than the third-highest solids-containing fluid in the survey. In fact, there are two tiers of values, the first being in the range of 1,000 lb/1,000 bbl, and the second being in the range of 600 lb/1,000 bbl. The B and S fields, of interest here, are in the second tier. Thus, the B and S fluids have "world class" solids content. As discussed below, this partially explains why the crude oil is so difficult to demulsify.
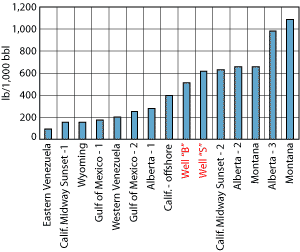 |
Fig. 1. Solids content of produced oil from the two fields of interest (B and S) compared with data from other locations of high-solids oil.
|
|
Solids are roughly composed of half silica fine sand particles and half scale compounds such as barium sulfate and calcium carbonate. The size distribution was broad, with at least half of the particles smaller than 10 microns. The fine sand particles are not necessarily an indication of imminent sand failure, and indeed they do not necessarily come from the near wellbore region. They are, however, typical of loose turbidite unconsolidated sands. For example, the daily test for sand production is carried out using a 150-mesh (100-micron) screen, and typical results are in the range of 0.0001 v/v% sand, which equals roughly 0.4 ppt. This is much lower than the previously stated result of 600 ppt that was found using the ASTM test with a 0.45-micron filter.
With regard to emulsions, the most important properties of solids are the size, the quantity (already discussed) and the surface-wetting characteristics. As a general rule, water-wet solids will stabilize an oil–in-water emulsion, and oil-wet solids will stabilize a water-in-oil emulsion. Many types of solids—particularly scale mineral precipitates, like sulfate and carbonate, and corrosion products, like iron sulfides—are initially water wet. As the produced oil and water mix in the wellbore, flowline and valves, various organic components of the produced fluid can adsorb on the water-wet solid surfaces and convert them to at least partially oil-wet solids. Such solids, particularly the small particles, are very effective at stabilizing water droplets in oil. Both produced oil and water contain such wetting agents, including short-chain organic acids, naphthenic acids, resins and asphaltenes, other polar compounds and waxes. Any amphoteric component that is itself moderately surface active has the potential for wetting the solid surface and enhancing its emulsion-stabilizing properties.
Literature data shows that at high concentrations of an oleophilic surface-wetting agent (e.g., asphaltene, wax, naphthenate, soap or fatty acid) added to a suspension of solid particles, the naturally water-wet solids become oil-wet and capable of stabilizing a water-in-oil emulsion, Fig. 2.2 In this case, the solids are initially water wet; therefore, at low concentration of the wetting agent, the solids stabilize an oil-in-water emulsion. As the concentration of the wetting agent increases, the solids become oil wet and the character of the emulsion changes from oil-in-water to water-in-oil.
 |
Fig. 2. At high concentrations of an oleophilic surface-wetting agent (e.g., asphaltene, wax, naphthenate, soap or fatty acid) added to a suspension of solid particles, the naturally water-wet solids become oil-wet and capable of stabilizing a water-in-oil emulsion.
|
|
As is the case with many waterfloods, the mingling of seawater and produced water in the formation will cause some scaling. With seawater breakthrough, barium and strontium sulfate particles can form. The particles can be very small (<1 micron to 2 microns), particularly in the presence of a scale inhibitor, which prevents scale depositing but does not prevent scale solids from precipitating. As the fluids flow from wellbore to topsides, both the temperature and the pressure decrease, which can allow sulfate minerals, as well as asphaltene, to precipitate. As asphaltenes precipitate, they may adsorb on available mineral surfaces. Such fine particles, with an asphaltenic coating, are excellent water-in-oil emulsifying agents. The smaller the particles are, the tighter the emulsion (i.e., the smaller the emulsion drops) will be. The greater the solids concentration is, the greater the fraction of emulsion will be. Such oil-coated particulate can also be dispersed into the water phase via shear during pressure drop or during pumping, thus contributing significantly to degradation of water quality.
Once a solid–stabilized emulsion forms, it is particularly difficult to separate the components. A fraction of the emulsion drops, if dispersed into the water phase, will be neutrally buoyant in the produced water. That is, depending on the relative quantities of oil and solids, the combination of a lighter-than-water component (oil) and a heavier-than-water component (solid) can result in a specific gravity close to that of water. When this occurs, separators and hydrocyclones will not provide effective separation because there is insufficient density difference to drive relative movement of the oil and water. A flotation unit can still be effective, since it uses the bouyancy of small gas bubbles to move the neutrally bouyant contaminants to the water"s surface, from which they can be collected and removed. It should be noted that, while flotation can be highly effective, its application is generally limited to low levels of oily contaminants (e.g., <300 ppm).
One particularly troublesome problem occurs when a flocculating water clarifier is used. In that case, a sticky polymer floc can form from the combination of oil, solids and polymer. When this floc is rejected back into the process stream, it can accumulate more solids and oil. This can result in more emulsion, thicker and more viscous pads in the separator vessels and fouling of vessel internals, including plugging of inlet headers, collection headers, distribution screens, treater grids and level indicators.
IP separator emulsion analysis. As discussed in Part 1, the intermediate-pressure (IP) separator receives fluid from three main sources: the primary separators, recycled oily water from the bulk oil treaters (BOTs) and recycled oily water from the closed-drain tank. Both oily water streams are sheared through a level control valve and are further sheared through a high-speed centrifugal pump. As discussed, the closed-drain tank is a catch-all for several streams having a wide range of properties from condensed water, gas condensate, oily rejects from the water treating system, and possibly from synthetic components such as lubrication oil and triethylene glycol. The main feed is the oily rejects of the hydrocyclone and the induced gas flotation unit. Since these fluids are sheared through a level control valve and a pump, they become mixed well and emulsified. The IP separator built up an interface emulsion pad on a frequent basis.
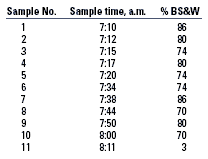
The field personnel observed that, every three or four days, the IP separator"s outlet BS&W would rise until it roughly equaled that of the feed. At that point, the operations personnel would drain the entire liquid inventory of the IP separator to the cargo tanks. The field personnel observed that the fluid drained out of the vessel was viscous.
With the help of the field personnel, we obtained fluid samples at regular intervals during one such draining exercise. We then measured the BS&W of those samples, Table 2. The first sample obtained (around 7:10 a.m.) corresponds to the bottom of the pad, which is closest to the water-oil interface, and the last sample obtained (around 8:11 a.m.) corresponds to the top of the pad, which is closest to the oil-gas interface. Based on estimates of the flowrate and information regarding the vessel geometry, the pad was roughly 2 ft thick. Thus, it occupied a large fraction of the liquid section of the vessel and significantly reduced the fluid residence time. The BS&W was relatively high throughout the pad, suggesting that it was composed mostly of water. However, as noted above, the pad fluid was very viscous and oily in appearance, which is typical of solids-stabilized emulsions.
| TABLE 2. IP separator pad sampling and BS&W results |
 |
|
In an optical microscope, the oil appeared to be the continuous phase in the pad. The emulsion had a wide range of drop sizes and a very high volume of dispersed phase (water) in a very small volume of continuous phase (oil). Also, the water drops appeared to have many small drops of oil dispersed within them. Thus, the emulsion was composed of small drops of oil dispersed within water drops, which were dispersed in a continuous oil phase.
Solvent tests were carried out for several pad samples to verify which phase was continuous. The pad samples were split into subsamples, to each of which a different solvent was added. Each sample with solvent was centrifuged and examined. A different solvent was then added to some samples, and those samples centrifuged again. One of the less ambiguous results was from the sample, from the bottom emulsion layer, to which xylene was added. The drop held together well and showed no mixing with the xylene, consistent with an oil-in-water-in-oil emulsion.
The results of the BOT and IP separator pad analysis, together with the fact that significant quantities of oily water are recycled from the BOT to the feed of the IP separator, lead to the following conclusions:
- Two emulsion-forming processes occurred in the system: a chemically and/or solids-stabilized, reverse (O/W) emulsion resulting from the closed-drain recycle and MeOH, and a normal W/O emulsion resulting from shearing and recycling
- The two pads stabilized each other
- The pads were thick, stable and viscous, thus reducing vessel efficiency
- The pads likely caused fluid to channel and prevent settling of any incoming BS&W.
A medium-term solution would be to provide a route for dumping the IP vessel"s contents that would not mingle those contents with the produced water stream. Preferably, the IP dump fluids would go directly to the off-spec cargo tank for subsequent slops treating. A long-term solution would be to address and eliminate the source of the emulsion pad, such as by improved chemical treating and reduction or elimination of problematic recycle streams. Both approaches are being pursued.
CHEMICAL TREATING
The chemical treating program was well managed. There was significant bottle-testing activity, resulting in identification of a new demulsifier that was optimized for fluids with high solids content. However, due to the remoteness of the facility, there was a significant delay in importing the chemicals needed for demulsification. Finally, in the later stages of this study, the new demulsifier was put onstream. It helped to resolve the pad in both the IP and the BOT. Other modifications, described below, helped by reducing the pad thickness to the extent that the pad did not cause a significant channeling effect. Thus, fluids entering the vessel had access to most of the liquid section of the vessel, resulting in increased retention time and significantly improved oil-water separation.
OPERATING PRACTICES
The operators were relatively experienced and had a strong chain of command. This resulted in general alignment across crews and in consistent operating practices, including strong process monitoring and record keeping. All things considered, the operating crew was one of the strongest components of the treating system on the FPSO.
SHORT-TERM IMPROVEMENTS
As discussed previously, the implementation of a demulsifier specifically developed for the treatment of solids-laden oil improved not only the dehydration of the oil, but also the treatment of produced water.3 Presumably, the demulsifier promoted the release of the solids from the oil-water interface. Since water quality improved, it is likely that some or most of the solids were discharged with the oil, although this was never determined.
Another major improvement was achieved by selection of water clarifiers for treating the off-spec water in the cargo tanks. As explained previously, the oily water became contaminated with seawater and sulfate-reducing bacteria. This resulted in the generation of hydrogen sulfide. Reaction of sulfide with dissolved iron in the produced water in the cargo tank formed iron sulfide, which is very insoluble. Rather than precipitate in a small number of large particles, iron sulfide precipitates in many small particles, which are easily oil wet and are one of the most effective stabilizers of oil-in-water emulsions. The presence of iron sulfide, oily solids and wave motion in the cargo tanks ensured that the off-spec produced water remained in an emulsified state.
However, once the presence of oily solids and iron sulfide was understood, the chemical vendor performed bottle tests and selected an appropriate set of water-treating chemicals. While importation problems restricted the available choices, suitable products were already available in the country. Furthermore, the cargo tanks containing off-spec water were treated with tetrakis (hydroxymethyl) phosphonium sulfate (THPS), a powerful biocide, to arrest the activity of sulfate-reducing bacteria. THPS also chelates iron sulfide.
The bottle tests indicated that the oil-in-water emulsion could be treated to some extent, the main benefit being flocculation of the solids-laden oil drops. The operations staff had a temporary skid constructed using readily available local equipment: a large impellor, low-specific–speed, centrifugal pump, sock filters and a bank of small-diameter hydrocyclones. The hydrocyclones were operated at a high DP ratio (reject DP divided by forward DP). The operation had to be conducted manually and would only process a small rate of water, but it was nevertheless a significant step forward.
Application of optimized chemicals significantly improved the performance of the water-treating system and made possible the use of a temporary treating skid to clean the water in the cargo tank. This is a case of applying a chemical approach to rescue a process-treating system that is not designed appropriately for the fluid characteristics.
LONGER-TERM IMPROVEMENTS
While the short-term improvements, especially the improved chemical program, increased the fluid-treatment capacity somewhat, there remained a substantial gap between the system capacity and the anticipated future water-production rate. With waterflood production, it is likely that water rates will at least double, and thus long-term improvements will be needed.
Several long-term recommendations were made, some of which have already been discussed. Long-term recommendations include installing pipe to allow more efficient draining of pads in the IP and BOT vessels, installing modified internals in the IP separator to reduce the high inlet velocity and increasing the feed pressure to the hydrocyclones to allow a higher DP ratio.
In addition, at least one of the cargo tanks should be converted to a modified slops-treating tank that will allow chemical treating, filtration if necessary, oil-water separation, routing of oil to the oil cargo tanks and routing of water to the water-treating system. This would provide a discharge route for those solids that are water wet and oil free.
In the primary separators, installing improved internals, such as perforated plates, would reduce sloshing dramatically. In addition, the interface level should be raised 1 ft, and the existing complicated internals should be modified to provide more uniform flow from the gas-liquid section to the liquid-packed section.
Finally, the closed-drain tank, which has been the source of so many problems, should be replaced with a significantly larger three-phase vessel, and the oil should be routed to the oil cargo tank. This would provide an additional discharge route for oily solids.
Several of these long term modifications are being implemented or are in the detailed design stage. 
LITERATURE CITED
1 Yang, C., Galbrun, M. and T. Frankiewicz, “Identification and resolution of water treatment performance issues on the 135D platform,” SPE 90409 presented at the SPE Annual Technical Conference and Exhibition, Houston, Sept. 25–29, 2004; Lee, J. and T. Frankiewicz, “In-field treatability test with the electrostatic susceptibility tester,” SPE 102221 presented at the SPE ATCE, San Antonio, Sept. 24–27, 2006. ; Frankiewicz, T. and J. Clemens, “Solving problems with overboard water handling systems,” World Oil, July 1999.
2 Tambe, D. E. and Sharma, M. M., “Factors controlling the stability of colloid-stabilized emulsions: An experimental investigation,” Journal of Colloid and Interface Science, 1993.
3 Poindexter, M. K., Chuai, S., Marble, R. A. and S. C. Marsh, “The key to predicting emulsion stability: Solid content,” SPE 93008, SPE Production & Operations, August 2006.
|
THE AUTHORS
|
|
John Walsh joined Shell in 1991 after obtaining a PhD in fluid phase equilibria at Johns Hopkins University. He joined Shell Exploration and Production Co. in 2001 as the chemical engineer for the Mars Tension Leg Platform. Since 2004 he has been involved in water treating problems across Western Hemisphere Shell assets.
|
|
| |
John Fanta, PE, has an MS degree in mechanical engineering from Rice University. During his 33 years with Shell, he has studied water treatment issues in a variety of locations including onshore and offshore California, the GOM and most recently in the Campos Basin, offshore Brazil.
|
|
| |
William Bryson is the subsea and topsides surveillance manager for Shell Brazil Exploration & Production. He previously worked for the company in the North Sea for 20 years on projects ranging from instrument engineering to installation management.
|
|
| |
Celso Toschi is a chemical engineer with an MS degree in process engineering. He has 27 years" experience in Campos Basin, five of which are with Shell Brazil on production chemistry application in the Bijupira and Salema offshore fields.
|
|
| |
Joseph Lee is director of Process Solutions at Natco Group Inc. He has 30 years" experience in the oil and gas industries with firms including Amoco, BP and Halliburton. He holds BS, MS and PhD degrees in chemical engineering. He can be reached at jlee@natco-us.com
|
|
| |
Ted Frankiewicz is a process engineering consultant with SPEC Services Inc. He has a PhD in physical chemistry from the University of Chicago. He has nearly 30 years" experience in oilfield processing, separations equipment design and produced water treatment. He can be reached at tfrankiewicz@specservices.com
|
|
| |
John Petty has been an engineering consultant to Modec International LLC for six years on the design and operation of the FPSO discussed in this article. He has 45 years of experience and holds BS in chemical engineering from Mississippi State University and an MS from Louisiana State University. He is a registered PE in Texas.
|
|
| |
Melvin Stacy graduated from Mississippi State University in 1963 as a mathematics major. He joined Baker Hughes in 1967 in production chemical applications and worked for Baker Hughes" water treating division from 1985 to 2001. Since then he has worked as a water treating specialist and consultant.
|
|
|